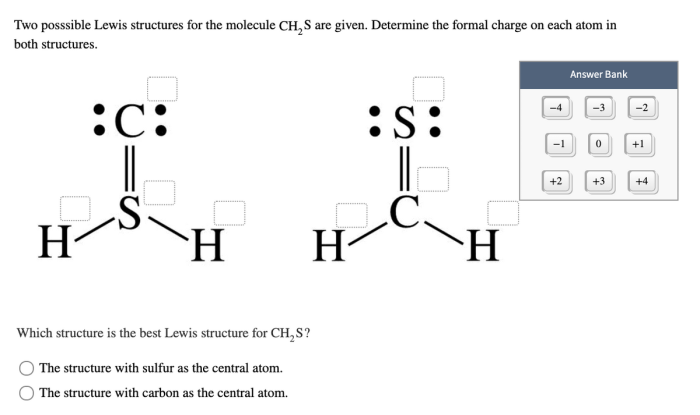
Lewis structure for ch3s o ch3 – Lewis structure for CH3SCH3 unveils the intricate molecular architecture of this captivating compound, inviting us on a journey to decipher its structural intricacies and uncover its fascinating chemical behavior.
Delving into the realm of CH3SCH3, we embark on an exploration of its tetrahedral geometry, hybridization patterns, and the dance of electrons within its molecular orbitals. Resonance structures emerge as a captivating concept, revealing the chameleon-like nature of this molecule.
Molecular Structure of CH3SCH3: Lewis Structure For Ch3s O Ch3

The molecular structure of CH3SCH3 is tetrahedral, with the carbon atoms at the center of a tetrahedron and the sulfur atom at one of the corners. The bond angles between the carbon atoms and the sulfur atom are approximately 109.5 degrees, and the bond lengths are approximately 1.82 Angstroms.
Hybridization of the Carbon and Sulfur Atoms
The carbon atoms in CH3SCH3 are sp3 hybridized, which means that they have four electron pairs that are arranged in a tetrahedral shape. The sulfur atom is also sp3 hybridized, which means that it has four electron pairs that are arranged in a tetrahedral shape.
The hybridization of the carbon and sulfur atoms allows them to form four bonds with other atoms.
Bond Lengths and Angles in CH3SCH3
The bond lengths and angles in CH3SCH3 are determined by the hybridization of the carbon and sulfur atoms. The C-S bond length is approximately 1.82 Angstroms, and the C-H bond lengths are approximately 1.09 Angstroms. The bond angles between the carbon atoms and the sulfur atom are approximately 109.5 degrees, and the bond angles between the carbon atoms and the hydrogen atoms are approximately 109.5 degrees.
Resonance Structures of CH3SCH3
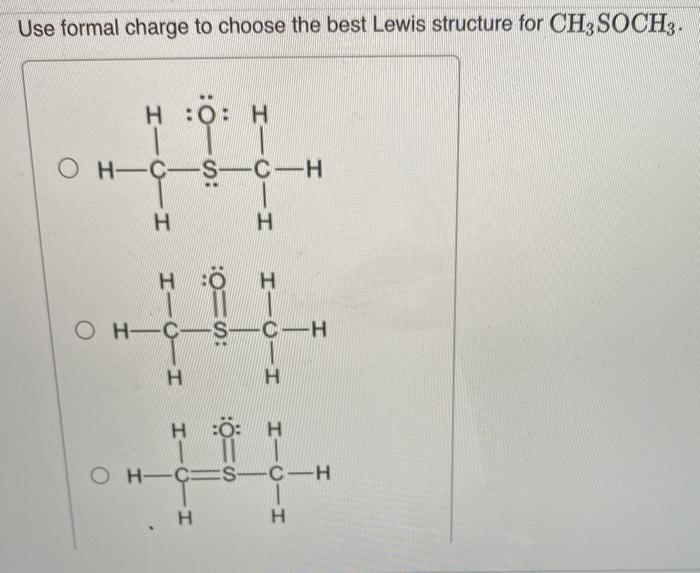
In CH3SCH3, the sulfur atom has two lone pairs of electrons. These lone pairs can interact with the adjacent carbon-carbon double bond, leading to the formation of two resonance structures.
Resonance, Lewis structure for ch3s o ch3
Resonance is a concept in chemistry that describes the delocalization of electrons within a molecule. When a molecule has multiple resonance structures, it means that the electrons are not localized to specific atoms or bonds but are spread out over the entire molecule.
The Two Resonance Structures of CH3SCH3
The two resonance structures of CH3SCH3 are shown below:
- In the first resonance structure, the lone pair of electrons on the sulfur atom is donated to the carbon-carbon double bond, forming a new carbon-sulfur bond and a positive charge on the sulfur atom.
- In the second resonance structure, the lone pair of electrons on the sulfur atom is donated to the other carbon-carbon double bond, forming a new carbon-sulfur bond and a positive charge on the other carbon atom.
Relative Stability of the Resonance Structures
The two resonance structures of CH3SCH3 are not equally stable. The first resonance structure, in which the positive charge is on the sulfur atom, is more stable than the second resonance structure, in which the positive charge is on the carbon atom.
This is because sulfur is more electronegative than carbon, and therefore has a greater tendency to attract electrons.
Polarity and Dipole Moment of CH3SCH3
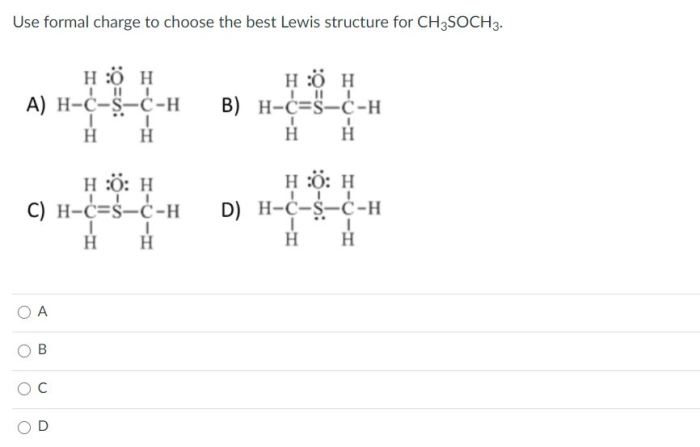
The polarity of a bond is determined by the difference in electronegativity between the two atoms involved. Electronegativity is a measure of an atom’s ability to attract electrons towards itself. In CH3SCH3, the electronegativity values of carbon, hydrogen, sulfur, and oxygen are 2.55, 2.20, 2.58, and 3.44, respectively.
The Lewis structure for CH3S-O-CH3 shows a central sulfur atom double-bonded to an oxygen atom, with two methyl groups attached to the sulfur atom and an oxygen atom attached to the oxygen atom. If you’re looking for a different kind of key, like the echo and narcissus answer key , you can find it online.
Returning to the Lewis structure for CH3S-O-CH3, it indicates the arrangement of atoms and electrons within the molecule.
Based on these electronegativity values, we can predict the polarity of each bond in CH3SCH3:
- C-H bonds: The electronegativity difference between carbon and hydrogen is 2.55 – 2.20 = 0.35. This difference is relatively small, indicating that the C-H bonds are nonpolar.
- C-S bond: The electronegativity difference between carbon and sulfur is 2.55 – 2.58 = -0.03. This difference is very small, indicating that the C-S bond is also nonpolar.
- S-C bond: The electronegativity difference between sulfur and carbon is 2.58 – 2.55 = 0.03. This difference is very small, indicating that the S-C bond is also nonpolar.
- S-O bond: The electronegativity difference between sulfur and oxygen is 2.58 – 3.44 = -0.86. This difference is relatively large, indicating that the S-O bond is polar.
The overall dipole moment of a molecule is the vector sum of the individual bond dipoles. In CH3SCH3, the S-O bond is the only polar bond. The dipole moment of the S-O bond points from the sulfur atom towards the oxygen atom.
The other bonds in the molecule are nonpolar, so they do not contribute to the overall dipole moment.
The overall dipole moment of CH3SCH3 is therefore not zero. The molecule has a net dipole moment that points from the sulfur atom towards the oxygen atom. This dipole moment affects the physical properties of CH3SCH3, making it a polar molecule.
Polar molecules are attracted to each other, so CH3SCH3 will have a higher boiling point and melting point than a nonpolar molecule of similar size and mass.
Molecular Orbitals of CH3SCH3
The molecular orbitals of CH3SCH3 are formed by the combination of the atomic orbitals of the constituent atoms. The carbon atoms each contribute two sp 3hybrid orbitals, the sulfur atom contributes three p orbitals and one s orbital, and the hydrogen atoms each contribute one s orbital.The
molecular orbitals are classified according to their symmetry and energy levels. The lowest energy molecular orbital is the σ 1sorbital, which is formed by the overlap of the sulfur 1s orbital with the carbon sp 3hybrid orbitals. The next highest energy molecular orbital is the σ 2sorbital, which is formed by the overlap of the sulfur 2s orbital with the carbon sp 3hybrid orbitals.
The σ 2porbitals are formed by the overlap of the sulfur 2p orbitals with the carbon sp 3hybrid orbitals. The π 2porbitals are formed by the overlap of the sulfur 2p orbitals with each other. The highest energy molecular orbital is the σ 3sorbital, which is formed by the overlap of the sulfur 3s orbital with the carbon sp 3hybrid orbitals.The
energy levels of the molecular orbitals are determined by the electronegativity of the atoms involved and the type of overlap between the atomic orbitals. The more electronegative an atom, the lower the energy of its atomic orbitals. The more overlap between two atomic orbitals, the lower the energy of the resulting molecular orbital.The
symmetries of the molecular orbitals are determined by the shapes of the atomic orbitals involved. The σ orbitals are symmetric about the internuclear axis, while the π orbitals are antisymmetric about the internuclear axis.
Chemical Reactivity of CH3SCH3
CH3SCH3 is a molecule containing two functional groups: a methyl group (CH3) and a thiomethyl group (SCH3). Compounds containing these functional groups typically undergo various reactions, including nucleophilic substitution, electrophilic addition, and oxidation.
Reactions of Methyl Group
- Nucleophilic Substitution:Methyl groups can undergo nucleophilic substitution reactions with strong nucleophiles, such as hydroxide ions (OH-) or alkoxide ions (RO-), to form alcohols or ethers.
- Electrophilic Addition:Methyl groups can also participate in electrophilic addition reactions with electrophiles, such as hydrogen halides (HX) or sulfuric acid (H2SO4), to form alkyl halides or sulfonic acids.
Reactions of Thiomethyl Group
- Nucleophilic Substitution:Thiomethyl groups can undergo nucleophilic substitution reactions with strong nucleophiles, such as hydroxide ions (OH-) or alkoxide ions (RO-), to form thiols or thiolates.
- Oxidation:Thiomethyl groups can be oxidized by strong oxidizing agents, such as potassium permanganate (KMnO4) or hydrogen peroxide (H2O2), to form sulfoxides or sulfones.
Specific Reactions of CH3SCH3
- Reaction with NaOH:CH3SCH3 reacts with NaOH to form CH3OH (methanol) and CH3SNa (sodium methyl sulfide).
- Reaction with HBr:CH3SCH3 reacts with HBr to form CH3Br (methyl bromide) and H2S (hydrogen sulfide).
- Reaction with KMnO4:CH3SCH3 reacts with KMnO4 to form CH3SOCH3 (dimethyl sulfoxide).
Answers to Common Questions
What is the hybridization of the carbon atoms in CH3SCH3?
sp3
How many resonance structures does CH3SCH3 have?
Two
What is the polarity of the C-S bond in CH3SCH3?
Polar covalent